Ease of Cutaquig SCIg Administration & Pharmacokinetics
An easy-to-use formulation
Low Viscosity Allows for Easy Injectability and Flexible Subcutaneous Administration1-3
- Low mean viscosity: 11.6 (± 0.6 )—Lower than 20% IgG products
- Progressive infusion speed
Adults
≥17 yearsChildren
2-16 yearsFirst 2 Infusions ≤20 mL/hour/site ≤15 mL/hour/site in children Increases in subsequent infusions 10 mL/hour/site (as tolerated) 5-10 mL/hour/site (as tolerated) in children Maximum 52 mL/hour/site (as tolerated) 25 mL/hour/site (as tolerated) in children
Find out more about cutaquig administration.
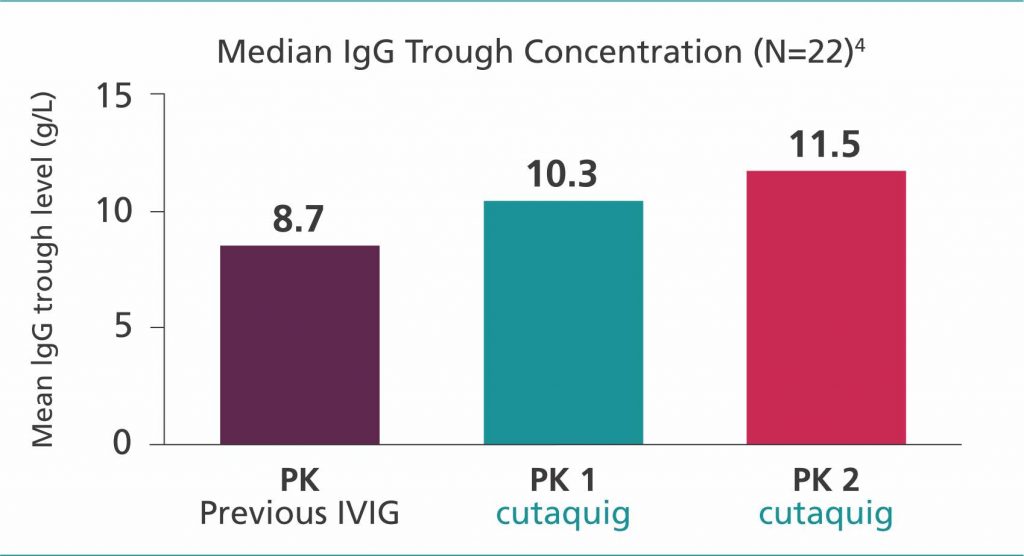
Favorable pharmacokinetics1,2,4,5
For both adult and pediatric patients 2 years of age and older, total IgG trough levels did not fluctuate during the course of the study, with higher trough levels achieved with cutaquig treatment compared with their previous IVIg
- At the end of the IVIg period, trough levels ranged from 5.0 to 5.1 g/L
- Over the entire SCIg treatment period, individual trough levels of total IgG ranged from 4.4 to 24.0 g/L
IgG: immunoglobulin G; IVIg: intravenous immunoglobulin; SCIg: subcutaneous immunoglobulin.
Once-weekly cutaquig infusions of approximately 161 mg/kg were:
- Sufficient to maintain trough IgG levels well above the minimum recommended level of 5-6 g/l.
- Correlated with low infection rates in children and adolescents with PI.5
In addition to once-weekly infusions, cutaquig offers a flexible dosing schedule that includes:
- Daily (2-7 days)
- Every other week
Dosing
| Switching from IVIg to cutaquig | Calculate using a conversion factor (1.30):
|
| Switching from other Subcutaneous Immunoglobulin (SCIg) | Dosing should be the same as for previous SCIg. |
| Flexible dosing schedule includes | Daily (2-7 days), Weekly and Every Other Week |
References:
- Kobayashi RH, Gupta S, Melamed I, et al. Clinical Efficacy, Safety and Tolerability of a New Subcutaneous Immunoglobulin 16.5% (octanorm [cutaquig®]) in the Treatment of Patients with Primary Immunodeficiencies. Front Immunol. February 2019 | Volume 10 | Article 40.
- Cutaquig Full Prescribing Information. Paramus, NJ: Octapharma; rev October 2021.
- Gelbmann N, Zöchling A, Mersich C et al. Cutaquig, Immunoglobulin (human) subcutaneous 16.5% solution for injection (165 mg/mL) – biochemical characterization, pathogen safety and stability. Presented at: 2018 Annual Meeting of the Clinical Immunology Society; Toronto, Canada. Poster Presentation.
- Octapharma, Data on File.
- Kobayashi RH, et al. Immunotherapy. 2021;13(10):813–824.